Johnson & Johnson pause means Hawaii clinics may offer alternative coronavirus vaccines or reschedule appointments
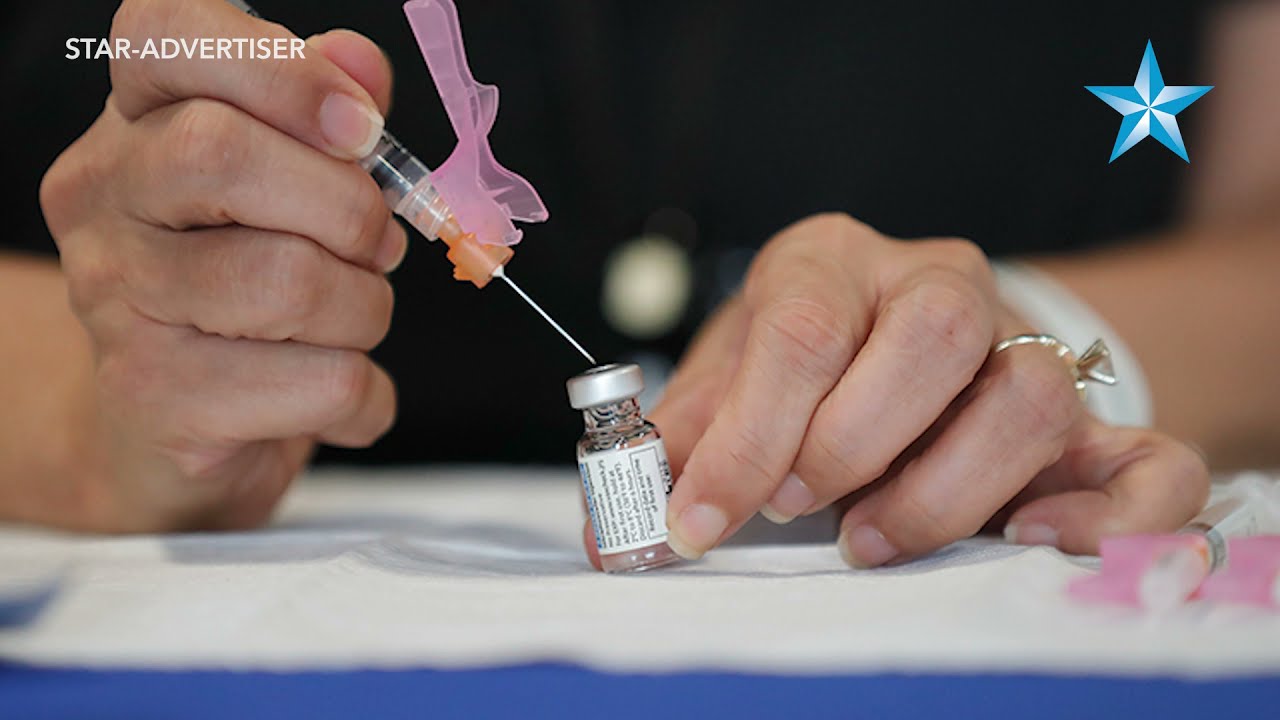

JAMM AQUINO / MARCH 9
VA pharmacy tech Wendy Joy prepares a dose of the Johnson & Johnson COVID-19 vaccine at Cloudbreak Community Housing in Barbers Point.


State Health Director Libby Char said that the pause on the use of the Johnson & Johnson vaccine will not affect meeting the presidential mandate on Monday to open up COVID-19 vaccination to all eligible.
“This will be a little bit of a setback for us, but we weren’t receiving that much Johnson & Johnson,” she said. “This week we were only on target to receive 2,600 doses. The J&J factor is not going to affect us greatly. We’re still counting on Moderna and Pfizer, and we’ll meet that presidential mandate of April 19.”
Vaccination clinics in Hawaii scheduled today to administer the Johnson & Johnson/Janssen COVID-19 vaccine may either offer the Moderna or Pfizer vaccine or reschedule the appointment, the Hawaii Department of Health announced today.
The Centers for Disease Control and Prevention and the Food and Drug Administration have recommended a pause in the use of the vaccine due to an extremely rare and severe type of blood clot found in six people after receiving the J&J vaccine.
All six cases affected women between the ages of 18 and 48, including one death, and symptoms occurred six to 13 days after vaccination.
As of Monday, more than 6.8 million doses of the J&J vaccine have been administered in the U.S., the CDC said today.
Don't miss out on what's happening!
Stay in touch with top news, as it happens, conveniently in your email inbox. It's FREE!
The pause is needed to prepare the health care system to recognize and treat patients appropriately.
Those who have received the J&J vaccine and who develop severe headache, abdominal pain, leg pain or shortness of breath within three weeks after vaccination should contact their health care provider, the CDC and FDA said in a joint statement.
Treatment of this specific type of blood clot using an anticoagulant drug called heparin may be dangerous, the CDC and FDA cautioned. Alternative treatments must be given, said Dr. Anne Schuchat, principal deputy director of the CDC, and Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
The rare type of blood clot, cerebral venous sinus thrombosis (CVST), was seen in combination with low blood platelet levels (thrombocytopenia), they said.




