Hawaii coronavirus saliva test could help turn pandemic’s tide, doctors say
CRAIG T. KOJIMA / CKOJIMA@STARADVERTISER.COM
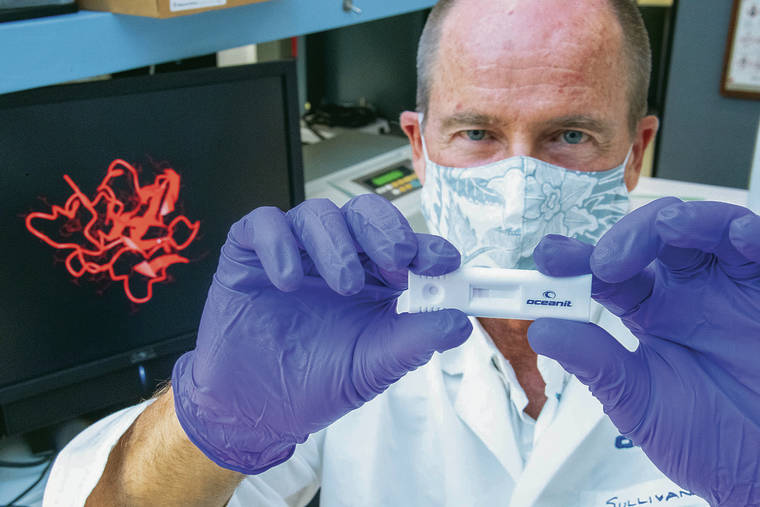
Oceanit CEO and founder Patrick Sullivan holds a small plastic tube resembling a pregnancy test that shows two lines if you are positive for the new coronavirus. On his computer screen is a picture of the protein inside the virus.

CRAIG T. KOJIMA / CKOJIMA@STARADVERTISER.COM
CEO and founder Patrick Sullivan and his team in the Oceanit Laboratories are developing a spit-in-cup COVID test.


Hawaii’s Oceanit Laboratories has developed a “spit in cup” test for SARS-CoV-2 Opens in a new tab, the virus that causes COVID-19, that can be done at home, gives results in three to 10 minutes and will cost about $20.
Within the next few weeks, The Queen’s Medical Center Opens in a new tab plans to start human clinical trials of the test, called ASSURE-19, having received a green light from its institutional review board last week, said Dr. Todd B. Seto, the center’s director of academic affairs and research.
Patrick Sullivan, CEO and founder of Oceanit Laboratories Opens in a new tab and author of “Intellectual Anarchy: The Art of Disruptive Innovation,” said a cheap, easy-to-administer, fast test is needed to open Hawaii’s economy.
Testing travelers for COVID-19 three days before their flights to Hawaii, and letting them bypass the state’s 14-day quarantine if the test is negative, as currently proposed by the state, won’t be enough to protect the community, Sullivan said.
“People can become infected after they’re tested, and can unwittingly infect other people before symptoms appear,” he said. “What’s needed is abundant and rapid testing, which is not available with the standard swab tests that are in short supply and often involve days of waiting for results.”
That’s why, as cases have rebounded and surpassed pre-lockdown numbers in Hawaii, Oceanit rushed to develop the spit-in-cup test that works much like a home pregnancy test.
Don't miss out on what's happening!
Stay in touch with breaking news, as it happens, conveniently in your email inbox. It's FREE!
The cost will be $20 per saliva test, Sullivan estimates, compared with $75-$170 per swab test in Hawaii, depending on where you’re tested and your insurance, as estimated by Dr. Scott Miscovich, an advocate of expanded testing who conducts tests at numerous sites.
Seto, of Queen’s, said, “We’re ready to go (with the human clinical trials), just working out the logistics. The (Queen’s institutional review) board met on an emergency basis because they recognized everything has to happen faster now, these times are different.”
A single test can be used by residents wanting a quick check, say, before going back to work or school, but there will be a required, multiday regimen for travelers, Sullivan said.
“The concept is for arriving passengers to get tested at the airport, and if you’re negative for the virus, you’re provided with five test kits plus a phone app, and you self-test for the next five days, which cover the average incubation period before symptoms appear,” he said.
“On average,” he added, “an infected person reaches peak contagiousness between Days Four and Six after exposure.”
According to the U.S. Centers for Disease Control and Prevention website, 50% of COVID-19 transmissions occur prior to symptom onset, and 40% of infections are asymptomatic.
Each day you test negative with Assure-19, Sullivan said, you’re free to go out, but if you test positive, you go into quarantine. “We call it the Aloha Protocol.”
By offering a voluntary alternative to the mandatory 14-day quarantine, he added, the protocol should spur a resurgence in visitors, for whom the $120 total cost for the six days of self- administered tests would be comparable to the price of a single swab test taken 72 hours before flying to Hawaii.
As an added safeguard, there’s built-in surveillance: “The app on your phone reads a QR code on the test and records the result,” Sullivan said.
Queen’s researchers will initially test at least 30 people for COVID-19 using both a swab test and Assure-19, compare results and give the data to the U.S. Food and Drug Administration.
“I love Patrick’s concept,” Miscovich said, adding that Oceanit’s alternative test was desperately needed now that “COVID-19 is exploding throughout the U.S.”
It reflects a broader shift in thinking about testing, Miscovich said. “The polymerase chain reaction swab test still is the gold standard because it’s the most sensitive and accurate in detecting the virus, but now we’re finding that if we test people in the peak phase of their infection, when the virus is replicating exponentially and they’re most contagious, these very large amounts of viral particles can be detected in a less sensitive saliva test.”
A test like Oceanit’s can help prevent transmissions because it provides quick results and can be given on a very large scale, said Roy Parker, a professor of chemistry and biochemistry at the University of Colorado at Boulder; co-author of an editorial, “Why we must rethink test sensitivity to contain COVID-19,” in The New England Journal of Medicine; and co-author of a forthcoming study comparing COVID-19 tests.
“What’s really important, in terms of a public health perspective, is to have a rapid test, which is not perfect, but good enough to say you might have COVID-19, so you can go get a (polymerase chain reaction) test and quarantine yourself until you get the results,” Parker said.
Several groups are developing saliva tests, he said.
On May 8 the U.S. Food and Drug Administration authorized the first diagnostic COVID-19 test, using home-collected saliva samples that then must be mailed to Rutgers Clinical Genomics Laboratory for results.
“In terms of being able to rapidly detect people who are infected that are asymptomatic, I’m really excited about (Sullivan’s) test because they use what’s called a DNA aptamer, which binds very tightly to part of the virus and is much easier to scale,” Parker said.
Miscovich said a saliva test’s lower sensitivity is actually a reason it can be more effective in detecting transmissible disease.
“The problem is, the PCR test is so sensitive, you can get a positive that will linger on and on and on and doesn’t reflect infectivity,” Miscovich said. “The inactive particles can still be detected in your system weeks after you’ve had an infection and are no longer contagious.”
In addition, Sullivan said, state involvement would likely be needed. “For visitors, I don’t think the protocol will work unless it’s part of an enforceable state policy that says you either do a 14-day quarantine or the Aloha Protocol.”
Edward Desmond, state Department of Health laboratories administrator, said he had read about the test on Oceanit’s website and was hopeful.
“There’s been a shortage of supplies to do the lab-based, molecular (PCR) testing as the case numbers go up on the mainland; three of our labs here in Hawaii have had a shortage,” he said. “It would be good if there was an alternative technology that would supplement our testing capacity. “
But he wondered how specific it would be in binding to the intended virus and not others. “Will it yield any false positives?”
“We estimate its specificity at about 97%,” Sullivan said. “This is considered very good, and should have very low false positives.”
If the Queen’s clinical trials are a success, he said, Oceanit will seek rapid approval from the FDA and to raise enough money to start manufacturing tests in Hawaii, stimulating the local economy and ensuring enough tests for island residents and visitors while distributing the rest worldwide.
In addition, the National Institutes of Health has selected Oceanit to compete in its $1.5 billion “Shark Tank”-esque Rapid Acceleration of Diagnostics competition aimed at accelerating the development of quick, accessible COVID-19 tests.
“It’s really exciting that Hawaii can be a leader in this,” Seto said, “and with all the business and research partners, it’s a nice example of a community collaboration to help ourselves get off the ground, locally.”
Sullivan said he hoped the collaboration would also build momentum toward a shift in the islands’ economy, where “instead of waiting for change to come from the outside, we use our imaginations and send innovation from Hawaii out to the world.”



